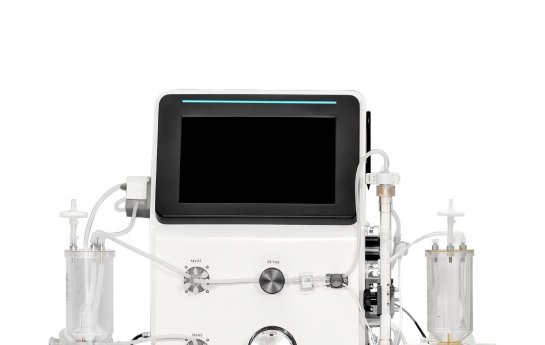
Antibody-conjugated drugs (ADCs) are novel tumor treatment drugs that possess both precise targeting and efficient killing capabilities. With advantages such as enhanced efficacy and reduced systemic toxicity, they have become a research hotspot. The tangential flow filtration (TFF) technology is widely used in the downstream process of ADC drugs, capable of efficiently removing organic impurities, coupling related impurities, and achieving buffer solution replacement. The ADC recovery rate exceeds 90%. This technology requires optimizing the process by controlling key parameters such as inlet and outlet pressure, flow rate, and pH. The core application lies in antibody concentration and desalination, product purification, and buffer solution replacement of ADC drugs, providing core support for the efficient preparation and stability of ADC drugs. Yocell's tangential flow system, through technological innovation, helps advance anti-tumor treatment towards precision and reliability.